6 Ways BROQ Is Better Than Before


20mg sulforaphane
per serving
25mg sulforaphane
per serving
Required refrigeration to maintain full potency
(At room temperature, lost 2% per month)
No need to refrigerate
Will maintain full potency for years
Stomach acid destroyed 30% of the
sulforaphane in every serving
Delayed-release capsule is absorbed in
intestine, bypassing stomach acid
(Biggest Challenge for Many Customers)
A significant proportion of people reported gastrointestinal issues
Delayed-release capsule is absorbed in
intestine, bypassing stomach acid
Although solvents are safe and approved by the FDA, many people prefer to avoid them
Minimally processed
100% natural
Magnesium Stearate
Although these are safe and approved by the FDA, many people prefer to avoid them
Just 3 plant-based ingredients
No artificial fillers

Original BROQ was the US brand name for the French product Prostaphane®
BROQ is no longer the same as Prostaphane®. We’ve made the following improvements:
- (1) 25% more sulforaphane
- (2) Much more stable, no damage from heat
- (3) More bioavailable (90-100%)
- (4) Eliminated stomach discomfort
- (5) Now made without solvents — all natural
- (6) No maltodextrin or magnesium stearate
- Now Made in the USA!
PRECURSORS ARE BETTER
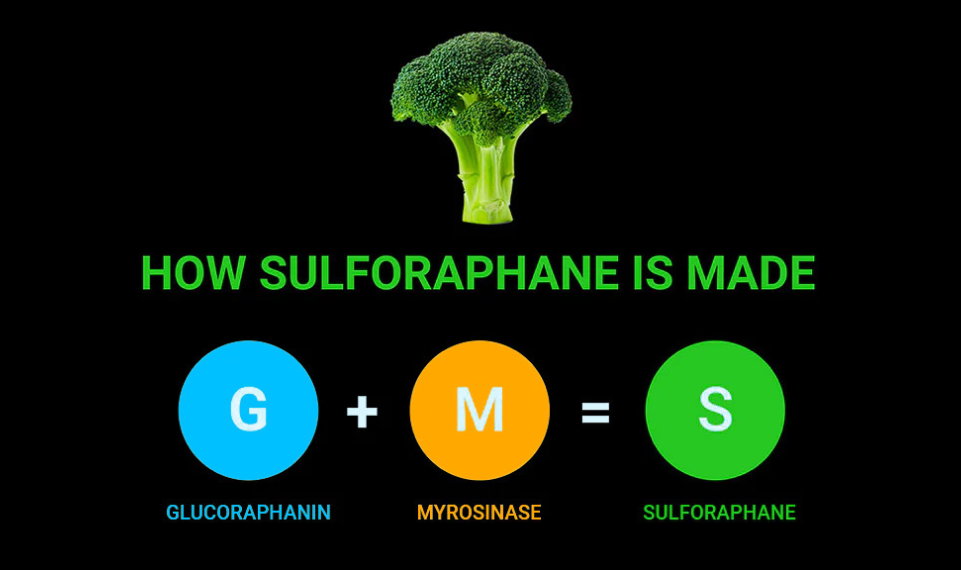
Broccoli does not contain sulforaphane. It contains glucoraphanin and myrosinase, the main building blocks (“precursors”) which combine to form sulforaphane in your body.

The False Assumption
A research finding from 30 years ago indicated that supplements containing sulforaphane were 70% bioavailable, versus 30% for ones containing the precursors, glucoraphanin and myrosinase.
What’s now clear is that those numbers were based on the effect of stomach acid on sulforaphane and its precursors.
The new 3rd-party lab tests (download full pdf) show that—after exposing sulforaphane to stomach acid—about 70% remains. But exposing supplements containing the two precursors to stomach acid reduces the resulting sulforaphane to 10 to 30%.
The simple solution is to use delayed-release capsules which do not release their contents until reaching the intestines, completely avoiding the acidic environment of the stomach.
The result is that virtually all of the sulforaphane is able to enter the bloodstream, since there is zero degradation due to stomach acid.
And the biggest advantage is that the precursors are highly stable and not affected by temperature.
A second advantage is that the precursors require far less processing, so you’re taking ingredients much closer to their natural state.
The Only One That’s Left...

The only product now sold in the U.S. that contains sulforaphane—rather than its precursors—is BrocElite®.
Unlike Original BROQ (and Prostaphane®), BrocElite® is produced without solvents and does not contain fillers. For those who object to the use of solvents and fillers, this is a plus.
When we first had a 3rd-party lab test BrocElite®, in 2021, the results (in water, pH 7.3) showed a sulforaphane content of 11.1mg per serving.
The new tests found a lower level: 2.4mg in water and 1.3mg in acid. (We were surprised by this and ran a 2nd test to confirm, with the same results.)
Their product does include other ingredients, which they claim have additional benefits beyond sulforaphane.
We do believe that BrocElite® is a high quality product, produced by a company that genuinely cares about improving the health of its customers.
The primary factor to consider in choosing which product to take is the amount of sulforaphane you will get from each serving.
Many of the human clinical studies of sulforaphane with the most impressive results have used dosages of 30-70mg sulforaphane per day.
New BROQ vs Original BROQ FAQs
What are the reasons that BROQ formulation was changed?
BROQ was already the most powerful sulforaphane supplement on the planet. We wanted to make it even better.
After four years of selling Original BROQ (the US version of the European product Prostaphane®), we had heard the following feedback from a significant number of customers:
(1) Digestive discomfort • This was the single biggest issue. Many people experienced gastrointestinal upset after taking Original BROQ.
(2) Instability, especially under high temperatures • Original BROQ lost 2% potency per month when stored at room temperature. When exposed to heat—for example, while in transit, in the hottest months of the year—the degradation is faster. We manufactured Original BROQ with enough extra sulforaphane to offset any likely loss of potency, but many people told us they did not like the lack of certainty about exactly how much sulforaphane they were getting.
(3) Maltodextrin & magnesium stearate • These inactive "filler" ingredients are safe and approved by the FDA, but many people prefer to avoid them.
(4) Solvents used in manufacturing • Every batch of original BROQ was tested to ensure there were no unsafe levels of artificial solvents remaining from the manufacturing. And, like the fillers, the use of solvents is safe and approved by the FDA. But many prefer to avoid even the smallest level of exposure to solvents.
--
Working with a US-based agricultural science company, we found a way to deliver a larger amount of sulforaphane per serving while completely avoiding the four issues listed above.
Does New BROQ have the same benefits as Original BROQ?
Yes.
Research indicates that all significant benefits of sulforaphane are dose-dependent, meaning that a greater benefit can be derived from a larger amount.
New BROQ delivers 25% more sulforaphane than Original BROQ.
Therefore, it is likely that the sulforaphane benefits from New BROQ should be on the order of 25% greater than those from Original BROQ.
Why is it better to take precursors than sulforaphane itself?
Taking precursors vs taking sulforaphane itself are equally effective.
If there were a supplement that delivered an equal amount (25mg) of sulforaphane as in BROQ — in a delayed-release capsule (released in the intestines rather than the stomach) — that supplement would be equally effective as BROQ.
However, such a supplement does not exist.
Neither Original BROQ nor Prostaphane® use delayed-release capsules.
Also — using precursors avoids the need for a complicated four-stage manufacturing process which relies on solvents.
With precursors, you are getting something much closer to its natural form.
Did the solvents and fillers present a danger to people who took Original BROQ?
No.
The trace amounts of solvents present in Original BROQ did not pose any danger.
Nor did the magnesium stearate or maltodextrin.
The CEO and Co-Founder of BROQ, Mike Franzini — who is highly dedicated to health optimization — personally took six capsules of BROQ per day for the past 5 years (since 1 year before its release).
--
Safety of magnesium stearate and maltodextrin in supplements
Magnesium Stearate: Widely Used and Well-Studied
Magnesium stearate is a commonly used additive in dietary supplements and medications, primarily as a flow agent to ensure consistent dosing and manufacturing. It is made from magnesium (an essential mineral) and stearic acid (a fatty acid found in foods like meat and cocoa butter).
Amount in Original BROQ Proven to be Safe: Studies show that magnesium stearate is safe when consumed in the small amounts present in supplements. The body readily metabolizes stearic acid, while the magnesium contributes to overall dietary intake (Toxicology Letters, 1996).
Not Linked to Immune Suppression: Some myths suggest magnesium stearate suppresses the immune system, but scientific studies have found no evidence to support this claim (Rowe et al., 2009).
Non-Toxic: Toxicology studies confirm that magnesium stearate is non-toxic at levels far exceeding typical dietary intake (Elder, 1984).
FDA Approved. Magnesium stearate is Generally Regarded As Safe (GRAS) by the FDA.
Maltodextrin: A Safe and Useful Excipient
Maltodextrin is a carbohydrate derived from starch (typically corn, rice, or potatoes) and is used as a stabilizer, filler, or carrier in supplements.
Readily Digestible: The human body breaks down maltodextrin into glucose, a natural energy source, through normal digestive processes. It has no harmful effects in the small amounts used in supplements (Anderson et al., 1999).
No Long-Term Risks: Maltodextrin has been used in food and supplements for decades without evidence of harm.
Will not affect blood sugar: While maltodextrin has a high glycemic index, the tiny amount present in a single serving of Original BROQ would likely have zero effect on blood sugar.
FDA Approved. Maltodextrin is Generally Regarded As Safe (GRAS) by the FDA.
References
Anderson, J. W., & Akanji, A. O. (1999). Carbohydrate digestibility and glycemic index. The American Journal of Clinical Nutrition, 70(3), 415–424.
Elder, R. L. (1984). Final report on the safety assessment of Stearic Acid. Journal of the American College of Toxicology, 3(5), 1–34.
Rowe, R. C., Sheskey, P. J., & Quinn, M. E. (2009). Handbook of Pharmaceutical Excipients. Pharmaceutical Press.
Toxicology Letters. (1996). Safety assessment of magnesium stearate. Toxicology Letters, 85(1–3), 1–8.
FDA. (2022). Overview of food ingredient safety. Food and Drug Administration.
Safety of Solvents Used in Supplement Manufacturing
Thorough Removal During Manufacturing
Solvents are commonly used during supplement manufacturing to extract, purify, or concentrate active ingredients. These include ethanol, water, and, in some cases, more specialized organic solvents. Manufacturers employ strict processes to ensure that solvents are thoroughly removed from the final product:
Regulated Residue Limits: Regulatory agencies like the FDA and European Medicines Agency (EMA) set strict limits on residual solvents. These are far below levels known to cause harm (ICH, 2021).
Evaporation During Production: Most solvents evaporate during the manufacturing process, leaving negligible amounts in the finished product. Advanced purification techniques, such as distillation or filtration, further ensure safety.
Approved Solvents Are Well-Studied
Solvents used in supplement manufacturing are selected for their safety profiles and are approved by regulatory authorities. Common examples include:
Ethanol: Widely used for botanical extractions, ethanol is the same alcohol found in beverages. Its low toxicity and ease of removal make it ideal for safe supplement production.
Water: As the most basic solvent, water is inherently safe and often used in combination with other solvents.
Hexane: Occasionally used for extracting plant oils, hexane residues in finished products are strictly controlled and removed to undetectable levels. Studies confirm that trace amounts within regulatory limits are non-toxic (Das et al., 2020).
Stringent Testing for Residual Solvents
Manufacturers are required to test products for residual solvent levels to ensure compliance with safety standards.
Good Manufacturing Practices (GMP): These regulations mandate rigorous quality control, including solvent residue testing, to protect consumers.
ICH Guidelines: The International Council for Harmonisation classifies solvents into three categories based on safety. Class 1 solvents (e.g., benzene) are prohibited, while Class 2 and 3 solvents are allowed only in minimal, non-toxic amounts (ICH, 2021).
Negligible Impact on Health
Residual solvents, when present at trace levels within regulatory limits, pose no meaningful risk to human health. They are far below thresholds known to cause harm, even with long-term exposure. This safety is supported by decades of use in food, pharmaceutical, and supplement industries.
References
Das, S., Roy, C., & Sen, G. (2020). Solvent extraction processes in the pharmaceutical industry. Journal of Applied Pharmaceutical Science, 10(4), 1–8.
ICH. (2021). ICH Q3C(R8): Impurities – Guidelines for Residual Solvents. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use.
FDA. (2022). Current Good Manufacturing Practice (CGMP) for Dietary Supplements. U.S. Food and Drug Administration.
European Medicines Agency (EMA). (2020). Guideline on the Use of Solvents in Pharmaceutical Manufacturing.
Why didn’t you use delayed release capsules in Original BROQ?
Original BROQ was the US version of the European product, Prostaphane®.
Prostaphane® had a long history and was always made with immediate-release capsules.
We deferred to the choice that had been made by the creators of Prostaphane®.
Why doesn’t every sulforaphane product use delayed release capsules?
Short answer: they should.
In the same way that we could have made the (better) choice to use delayed-release capsules for Original BROQ—but chose not to—the vast majority of supplement manufacturers choose not to, since it's highly unusual (especially until very recently) to use delayed release capsules for supplements.